Feb. 6th, 2026
1. Is Your Plasma Torch Really a "Star in a Jar"? Decoding the Core Physics
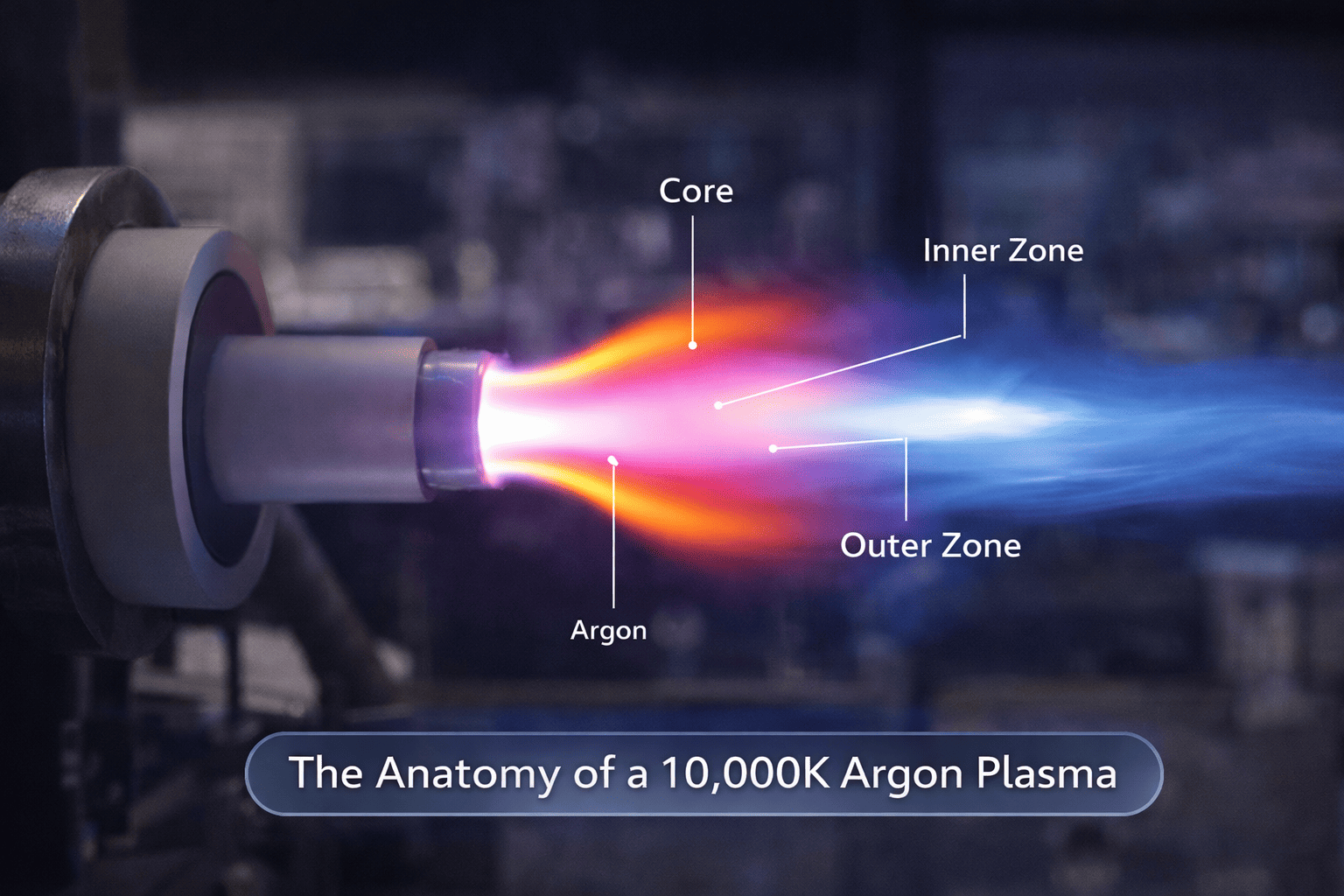
When a new analyst walks into my lab and asks, "How does ICP-OES work?" I usually tell them to step away from the software screen and look at the torch. As you can see in the "Anatomy of a 10,000K Argon Plasma" (Image 1), we aren't just running a machine; we are managing a controlled, high-energy state of matter.
ICP-OES (Inductively Coupled Plasma Optical Emission Spectrometry) is the art of stripping an element down to its bare soul. We use an RF (Radio Frequency) generator to kickstart a flow of argon gas into a high-energy state. We aren't just "heating" a sample; we are subjecting it to temperatures—roughly 6,000K to 10,000K—that are hotter than the surface of the sun. At this temperature, chemical bonds don't just break; they cease to exist. Every molecule in your liquid sample is ripped apart into individual atoms and ions.
1.1 What Really Happens During the Excitation and Relaxation Loop?
The "Optical Emission" part is where the data happens. In the central channel of the plasma torch, these excited atoms relax. To return to their ground state, they must shed energy, and they do this by emitting light at very specific, discrete wavelengths. Each element has a unique spectral signature. My job, as an expert, is to ensure the detector catches those photons without interference.
Understanding this process is fundamental to laboratory work. For those coming from a chromatography background, you might find our
guide on HPLC vs LCMS
helpful in understanding how different detection principles affect data quality.
2. Why Is the Path From Nebulization to Detection So Critical for Success?
The journey of a sample in an icp-o optical emission spectrometry chemical test begins long before the plasma. It starts with sample introduction, and this is where 90% of lab errors occur.
2.1 Could High-Purity Argon Gas Be the Missing Key to Your Data Accuracy?
One thing the sales brochures rarely emphasize enough is that your results are only as good as your gas. Argon gas purity is your baseline. If you are using 99.9% pure argon instead of 99.999% (five-nines), you aren't just saving money—you’re inviting ghosts into your data. Contaminants in the gas lead to "spectral interference" or a noisy baseline, which directly impacts your trace element analysis.
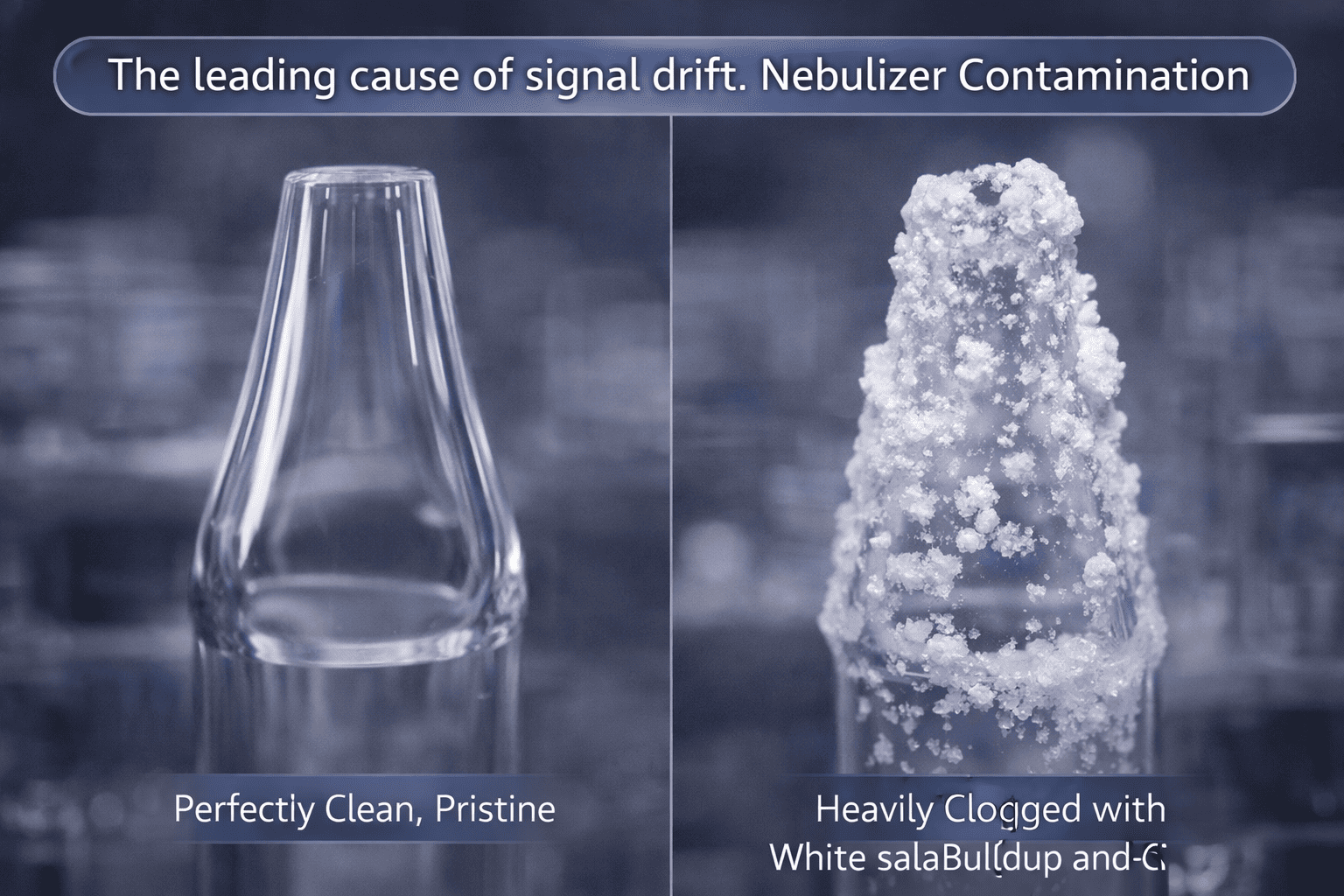
2.2 Is Nebulizer Contamination Quietly Sabotaging Your Lab’s Productivity?
As shown in Image 2 ("The leading cause of signal drift"), the difference between a pristine nebulizer and one with salt buildup is the difference between a successful run and a week of troubleshooting. The nebulizer is the most sensitive 10 centimeters of the entire instrument. Its job is to turn a liquid into a "fine mist" (aerosol).
Nebulizer contamination occurs when you pump complex matrices through a tiny orifice. If the droplets are inconsistent, your RSDs will skyrocket. If you aren't practicing rigorous sample filtration for ICP, you are essentially playing Russian Roulette with your uptime. For most aqueous samples, I highly recommend using high-quality
universal laboratory syringe filters
before the sample ever reaches the autosampler.
3. How Can You Effectively Manage Sample Stability in Complex Matrices?
In a high-throughput lab, the way you store your samples after digestion is just as important as the analysis itself.
3.1 Is Your Laboratory Equipped for the Brutality of Hydrofluoric Acid (HF)?
In mining or geochemical labs, hydrofluoric acid resistance is a necessity. The storage of these samples requires robust materials that won't leach silica. For large-volume storage and environmental digestion, we often rely on
24-400 EPA Vials for borosilicate glass sample storage
, which offer the chemical stability required for long-term trace analysis.
3.2 Are You Protecting Your Samples from Hidden Cross-Contamination?
While the ICP is firing, the next 100 samples are waiting in the rack. I have seen countless runs ruined because of poor sealing. Using high-quality
bonded caps and septa
is standard practice in my lab to ensure no volatile contaminants enter or leave the vials during long sequences.
4. Which Analytical Strategy Does Your Lab Truly Need: ICP-OES or ICP-MS?
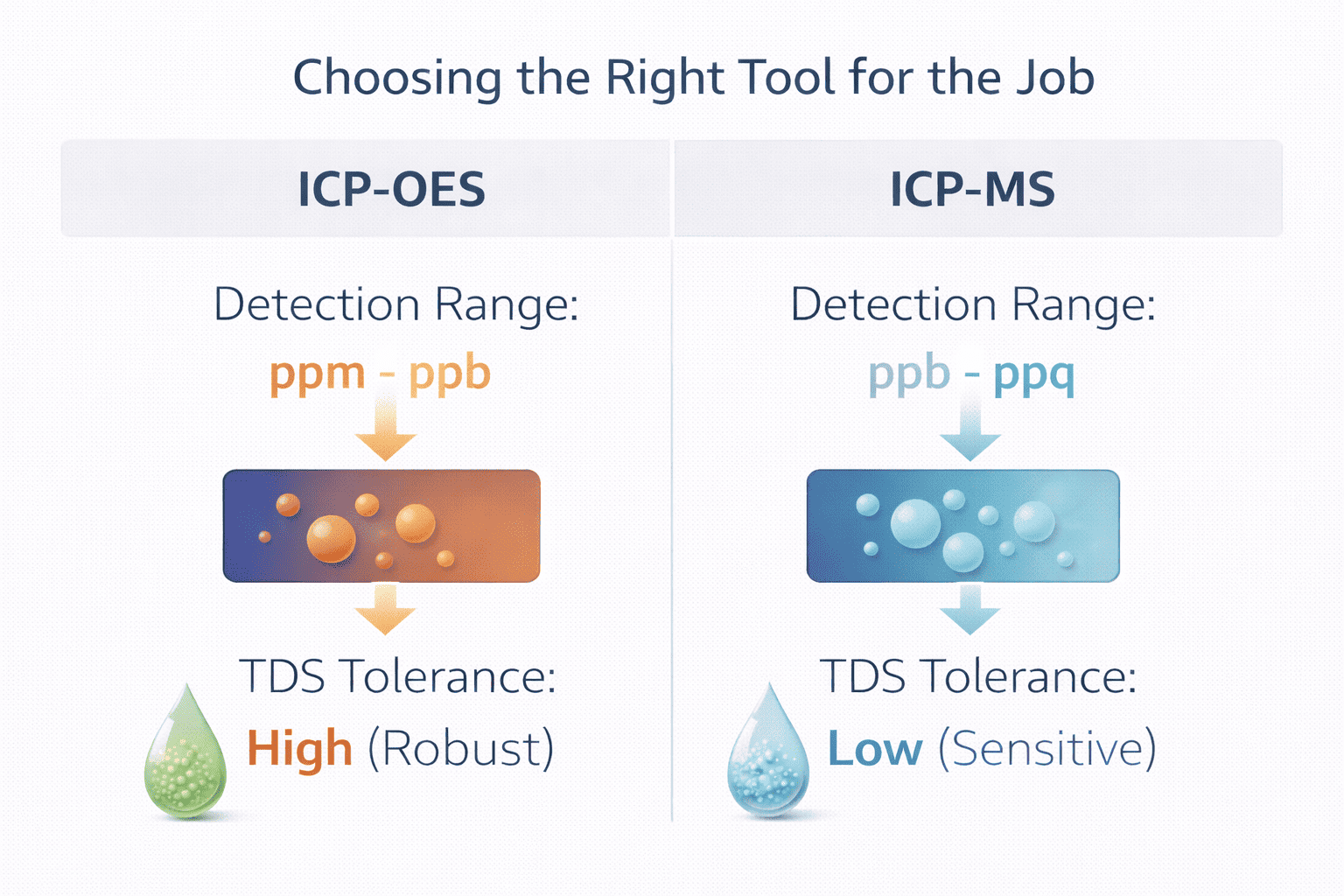
One of the most frequent questions I get from procurement officers is about the difference between icp-ms and icp-oes. Referencing the Technical Infographic (Image 3), it’s clear that the decision isn't about which machine is "better," but which is "correct" for your matrix.
4.1 When Is ICP-OES the Superior Choice as a Robust Lab Workhorse?
-
Robustness: ICP-OES has high "TDS Tolerance," handling up to 30% solids.
-
Cost-Efficiency: Lower operational costs and fewer delicate parts.
-
Speed: Ideal for multi-element analysis in mineral and waste oils (ppm range).
4.2 Is It Time to Upgrade to the Extreme Precision of ICP-MS?
If your analysis requires parts-per-trillion (ppt) levels, ICP-MS is your only choice. However, it is "Sensitive" and has "Low TDS Tolerance." For more on precision filtration to protect such sensitive systems, check this
guide to 0.22 micron filters
.
5. How Does an Expert’s Checklist Boost Your Lab’s Daily Reliability?
After two decades at the bench, I've learned that the "magic" of the plasma torch only works if the "mundane" tasks are handled with precision.
5.1 How Do You Achieve Absolute Consistency in Sample Introduction?
Whether you are using screw thread vials or
ND11 crimp neck vials
, the integrity of the seal is paramount. A failing seal leads to evaporation, which ruins your calibration curve.
5.2 Are "Ghost Signals" Ruining Your Results? Advanced Troubleshooting Tips
Sometimes, you get a signal for an element that shouldn't be there. Before you blame the instrument, look at the physical environment. If you are measuring low-level Sodium or Boron, cheap glass can leach these elements into your sample. Switching to high-purity polypropylene or specialized shell vials can often eliminate these "ghosts."
6. Can the Synergy Between Science and High-Quality Supplies Transform Your Results?
Understanding how ICP-OES works is a journey through plasma physics, but keeping it working is a matter of disciplined lab management. While the plasma torch is the star, it’s the supporting cast—the vials, the filters, and the caps—that ensure the data remains accurate.
Need Technical Assistance?
Struggling with signal drift or sample contamination?
Email:
boonemi@aijirenvial.com
WhatsApp:
+86 18338832256
.png)


 English
English
 Chinese
Chinese

.png)
