Feb. 4th, 2026
In the modern analytical laboratory, chromatography is far more than a routine protocol; it is an intricate dance of molecular interactions. Whether you are quantifying pharmaceutical impurities or detecting environmental pollutants at trace levels, understanding the fundamental physics of your separation is what distinguishes a technician from a master chromatographer.
Success in the lab doesn't just come from high-end instrument settings. It lives in the synergy between your method chemistry and the high-quality consumables that protect your sample integrity. For those navigating the complexities of
HPLC vs LC-MS: Which to Choose
, or struggling with unstable baselines, this guide shares the bench-side secrets to achieving world-class data and maximizing instrument uptime.
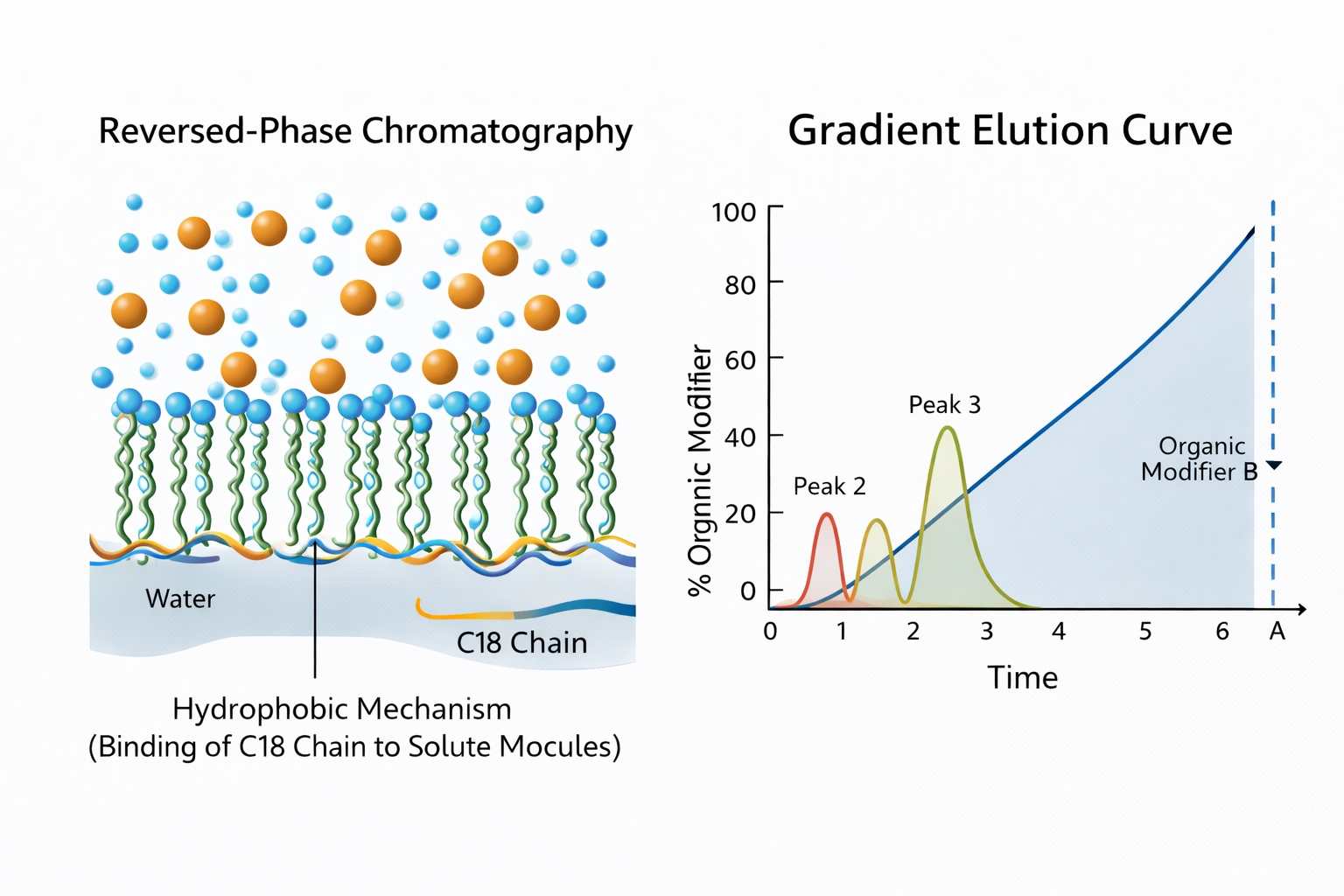
Understanding the Hydrophobic Handshake: What is Reverse Phase Chromatography?
To answer the foundational question—what is reverse phase chromatography—one must look beyond the textbook definition. At its core, it is a separation based on the "fear of water" (hydrophobicity). In reverse phase high pressure liquid chromatography (RP-HPLC), we utilize a non-polar stationary phase and a polar mobile phase to sort molecules by their hydrophobic character.
The Chemistry of the C18 (Octadecyl) Chain
The most common stationary phase involves silica-based particles bonded with C18 (octadecyl) chains. From my experience, the density of these C18 chains and the quality of the silica support determine your method's ruggedness. Many analysts encounter peak tailing when analyzing basic compounds. This is often caused by residual silanol groups on the silica surface acting as unwanted ion-exchange sites.
To solve this, we use an end-capped column, where smaller silanes "mask" these active sites. However, even the best column can't fix a poor injection. If your chromatographic peak looks distorted before it even hits the column, check your vial. Using high-purity
9mm Short Thread Vials with Universal Fit
ensures that your analyte doesn't adsorb to the vial walls before the needle even picks it up.
The Art of the Organic Modifier in HPLC Method Development
The retention time of your analytes is governed by the organic modifier HPLC concentration. By adjusting the ratio of Acetonitrile or Methanol to your aqueous buffer, you tune the "elution strength" of the mobile phase.
A common pitfall I see in method transfer is a misunderstood gradient elution curve. If you ramp your organic modifier too quickly, you lose resolution; too slowly, and you suffer from peak broadening and wasted solvent. When shifting between
Analytical vs Preparative HPLC
, managing the gradient slope becomes the primary factor in successful scale-up. Remember, Acetonitrile offers low viscosity and high elution strength, while Methanol can provide different selectivity for polar compounds that ACN might miss.
Deciphering GC Elution Order: Predicting Compound Separation
Predicting gc elution order is one of the most rewarding challenges for a gas chromatographer. Unlike HPLC, where solvent chemistry is the primary lever, GC separation is dictated by the "Trinity of Separation": boiling point, molecular polarity, and column temperature programming.
Thermodynamics vs. Dipole Interactions
In a non-polar stationary phase, the gc elution order strictly follows the boiling point. The more volatile compounds exit first. However, the game changes when you use a polar column like PEG/Wax. I recently handled a project involving isomers where the boiling points were nearly identical. By switching to a polar phase, the dipole-dipole interactions allowed us to separate these compounds based on their electronic structure rather than their size.
For high-sensitivity work like environmental trace analysis, the integrity of your sample container is paramount. Using
20mm Crimp Top Headspace Vials
prevents the loss of volatile analytes, ensuring that your mass spectral deconvolution is based on a true representation of your sample, not a leaked fraction.
HPLC vs LC-MS: Navigating the Sensitivity Shift
A frequent question in our lab is: "When should we move from HPLC vs LC-MS?" The answer lies in your required sensitivity. While HPLC with UV detection is excellent for routine QC at microgram levels, LC-MS is required when you need to detect picogram levels or identify unknowns in complex matrices.
If you are moving to LC-MS, your choice of vials becomes even more critical. Standard caps can leach plasticizers into your mobile phase, creating "Ghost Peaks" that haunt your chromatogram. This is why Aijiren’s
Bonded Screw Caps
are a staple in MS-certified labs—they eliminate the risk of the septa falling into the vial or leaching contaminants into the detector.
The Anatomy of a Perfect Chromatographic Peak
Every chromatographic peak is a diagnostic tool. A sharp, symmetrical Gaussian peak tells you that your system is optimized. A "shoulder" or "tail" tells you that something is wrong, often related to hardware or dead volume.
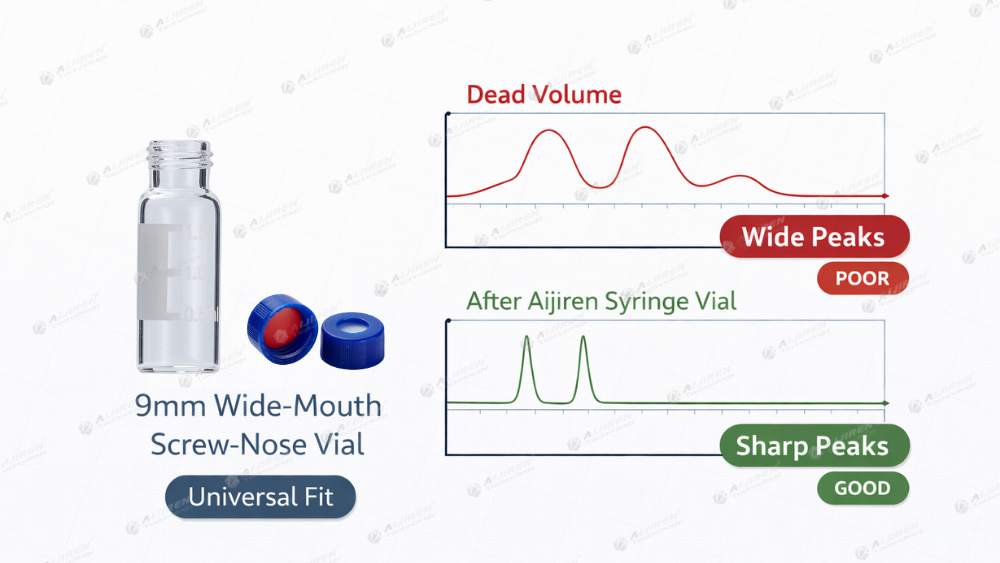
Eliminating Dead Volume and Extra-Column Volume
One of the silent killers of resolution is dead volume. If there is any gap between your needle and the bottom of the vial, the sample disperses. For micro-sampling, I always recommend using Conical Inserts. These inserts force the sample into a narrow, vertical path, ensuring a "sharp plug" injection. This directly results in a sharper peak and better signal-to-noise ratios, especially in high-pressure systems where dispersion is the enemy.
Troubleshooting Like a Pro: From Baseline Noise to Septa Coring
In my years of troubleshooting, I’ve found that 70% of instrument issues are actually consumable issues.
-
Baseline Noise & Ghost Peaks: If you see peaks where there should be none, your filters might be the culprit. Always refer to
The Complete Guide to 0.22 Micron Filters
before choosing your syringe filters.
-
Septa Coring: If you see bits of silicone in your vial, your needle is "coring" the septa. This happens with poor-quality caps. Our ND11 Crimp Caps and 10-425 Screw Caps are precision-engineered to withstand multiple punctures without fragmentation.
-
Stability in Storage: For EPA methods or long-term storage, using
24-400 EPA Vials
made of borosilicate glass ensures zero solvent loss and zero contamination.
Why Data Integrity Starts with the Consumable
We often spend 50,000 USD on a high-end system, then try to save pennies on the vial. In my experience, a sub-par vial is the most expensive thing in your lab because it leads to "re-runs" and "failed validations."
Whether you need 1ml Shell Vials for simple chromatography or 18mm Screw Thread Headspace Vials for automated GC, Aijiren provides the consistency that experts demand. We ensure that your gc elution order is reproducible day after day, and your chromatographic peak stays as sharp as the day you developed the method.
Expert Support & Inquiries: Are you facing inconsistent results or struggling with method development? Let’s optimize your workflow together. Contact me for a technical consultation:
Conclusion Mastering what is reverse phase and mastering the nuances of your instrument is a journey of continuous learning. By choosing the right organic modifier HPLC and best-in-class consumables, you are investing in the truth of your data. Don't let a sub-par vial ruin a brilliant method you spent weeks developing.


 English
English
 Chinese
Chinese


